3D matrix of mesenchymal stem cells (MSC)

Autologous MSC transplants are used to treat ischaemic cardiac syndromes. The functional recovery of the organ is mainly linked to paracrine effects, and therefore to the cell survival rate after implantation. However, current injection techniques only offer a low cell survival rate at three days, limiting the effectiveness of this therapeutic strategy.
COMPETITIVE ADVANTAGES
- Compatible with in vivo use (biocompatible, biodegradable, GMP-compatible ingredients and processes)
- Adaptable and reproducible functionality (control of 3D architecture and porosity)
- Easy handling (mechanical resistance)
APPLICATIONS
- In vitro cell support and culture
- Paracrine cell therapy, particularly in the cardiac field
INTELLECTUAL PROPERTY
- Patent
DEVELOPMENT STAGE
Laboratory validation of the technology
LABORATORIES

CIRIMAT and I2MC
Description
A 3D matrix of alginate and chitosan complexes that can be seeded with MSCs and makes it possible in vitro to:
- Conserve the cellular phenotype
- Maintain MSC viability at around 80% after one month
- Conserve or even increase the paracrine secretory capacity of MSCs compared with 2D cultures
A GMP-compatible manufacturing process for the matrix, enabling the architecture to be fully controlled
In vivo properties: excellent biocompatibility, significant improvements in cardiac function and neovascularisation of the infarcted zone, limitation of ventricular fibrosis.
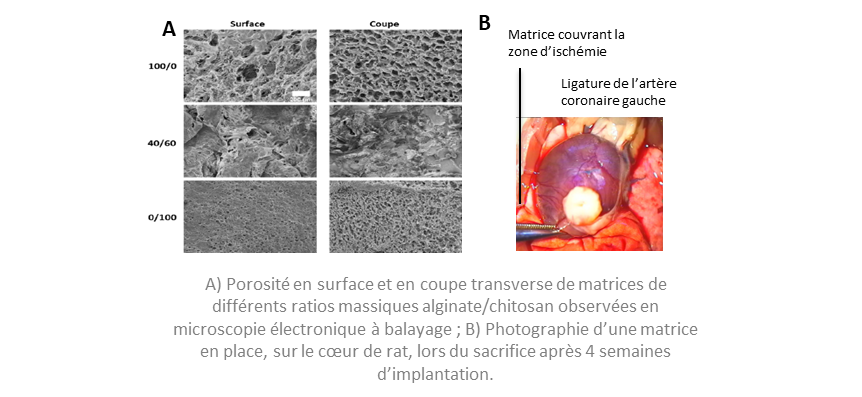
Technical specifications
- Pore size: 0.2 µm to 400 µm
- Pore volume: 60% to 98% of total volume
- Elastic modulus at 50% deformation: 1 to 100 kPa



